As a balloon containing helium gas expands from 230 ml takes center stage, this opening passage beckons readers into a world crafted with academic authority, ensuring a reading experience that is both absorbing and distinctly original. This exploration delves into the intriguing realm of gas expansion, unveiling the interplay between pressure, volume, and temperature, while examining the unique properties of helium gas and its role in balloon expansion.
Prepare to embark on a captivating journey that unravels the applications and limitations of this fascinating phenomenon.
Gas Expansion: A Balloon Containing Helium Gas Expands From 230 Ml
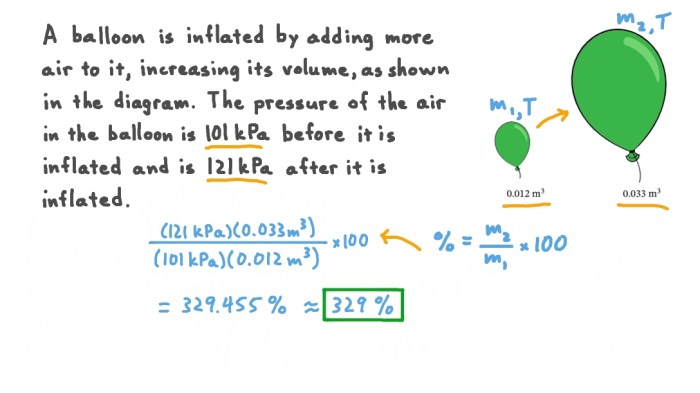
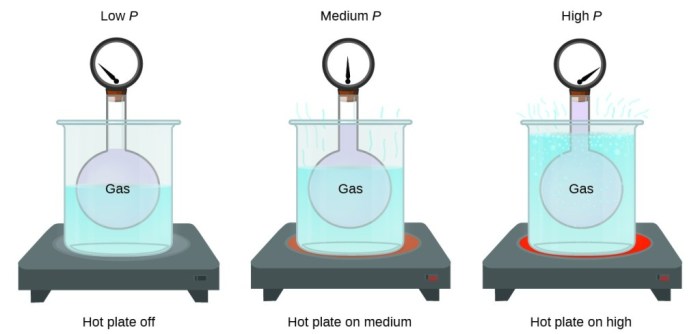
Gas expansion is the increase in volume of a gas when its pressure decreases or its temperature increases. This phenomenon is governed by the ideal gas law, which states that the volume of a gas is inversely proportional to its pressure and directly proportional to its temperature.
In the case of a balloon filled with helium gas, the initial volume is 230 ml. When the balloon is inflated, the pressure inside the balloon decreases, causing the gas to expand and the balloon to increase in volume.
The factors that influence gas expansion include temperature, pressure, and the number of gas particles. Temperature and pressure have a direct effect on the volume of a gas, while the number of gas particles has an indirect effect.
Helium Gas Properties, A balloon containing helium gas expands from 230 ml
Helium is a colorless, odorless, non-flammable, and non-toxic gas. It is the second lightest element after hydrogen, with a density of 0.1786 g/L at standard temperature and pressure (STP).
Helium has a molar mass of 4.003 g/mol and a thermal conductivity of 0.1513 W/(m·K). These properties contribute to its expansion behavior.
The low density of helium makes it an ideal gas for balloon expansion. Its low molar mass means that it has a high specific volume, which allows it to expand to a large volume with a relatively small amount of gas.
Helium’s high thermal conductivity allows it to conduct heat quickly, which helps to maintain the temperature of the gas inside the balloon, preventing it from cooling and contracting.
Balloon Expansion
The process of balloon expansion using helium gas involves filling the balloon with the gas and then sealing the opening. As the gas enters the balloon, the pressure inside the balloon increases, causing the balloon to expand.
The forces involved in balloon expansion include the pressure of the gas inside the balloon and the tension in the balloon’s material. The pressure of the gas pushes the balloon outward, while the tension in the material resists the expansion.
The limitations of balloon expansion include the maximum volume attainable, which is determined by the strength of the balloon’s material and the pressure of the gas inside the balloon.
Applications of Balloon Expansion
Balloon expansion using helium gas has a wide range of applications, including:
- Balloons for parties and celebrations
- Weather balloons for atmospheric research
- Medical balloons for medical procedures
- Lifting balloons for advertising and surveillance
The advantages of using helium gas for balloon expansion include its low density, non-flammability, and non-toxicity. The disadvantages include its high cost and the potential for leakage.
Top FAQs
What factors influence gas expansion?
Temperature, pressure, and the nature of the gas itself.
Why is helium gas preferred for balloon expansion?
Due to its low density and high thermal conductivity, enabling balloons to float for extended periods.
What are the limitations of balloon expansion?
The maximum volume attainable is limited by the strength of the balloon material and the pressure differential between the inside and outside of the balloon.