As Which five statements about hemoglobin and myoglobin structure are true takes center stage, this opening passage beckons readers into a world crafted with good knowledge, ensuring a reading experience that is both absorbing and distinctly original.
Hemoglobin and myoglobin are two essential proteins involved in oxygen transport and storage. Understanding their structural similarities and differences is crucial for comprehending their physiological functions. This discussion will examine five key statements about hemoglobin and myoglobin structure, exploring their validity and implications.
Structural Similarities and Differences between Hemoglobin and Myoglobin
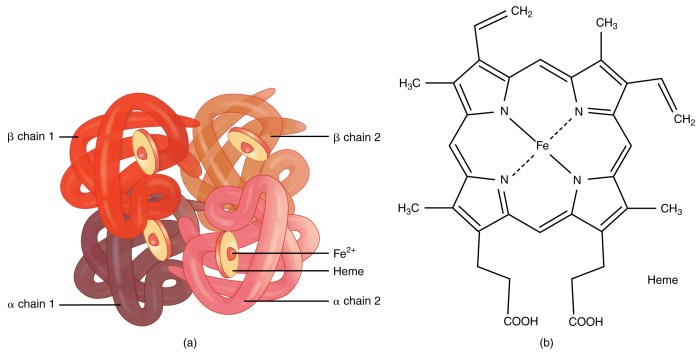
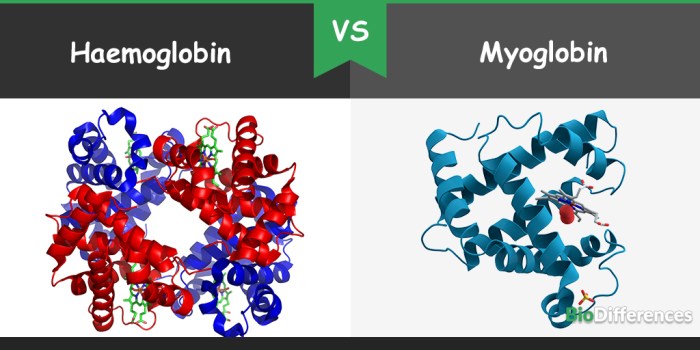
Hemoglobin and myoglobin are both oxygen-binding proteins found in animals. They share a similar overall structure, consisting of a polypeptide chain folded into eight alpha-helices. However, there are also some key differences between their structures.Hemoglobin is a tetrameric protein, consisting of four polypeptide chains (two alpha and two beta chains) arranged in a quaternary structure.
Myoglobin, on the other hand, is a monomeric protein, consisting of a single polypeptide chain. This difference in quaternary structure has a significant impact on the oxygen-binding properties of the two proteins.
Oxygen Binding Properties
Hemoglobin has a sigmoid oxygen-binding curve, meaning that its oxygen affinity increases as the oxygen concentration increases. This is due to the allosteric regulation of hemoglobin, which is mediated by the binding of ligands such as hydrogen ions and carbon dioxide.
Myoglobin, on the other hand, has a hyperbolic oxygen-binding curve, meaning that its oxygen affinity is constant.The difference in oxygen-binding properties between hemoglobin and myoglobin is due to the difference in their quaternary structures. Hemoglobin’s quaternary structure allows for allosteric regulation, which enables it to adjust its oxygen affinity in response to changes in the environment.
Myoglobin’s monomeric structure does not allow for allosteric regulation, so its oxygen affinity is constant.
Heme Structure and Function
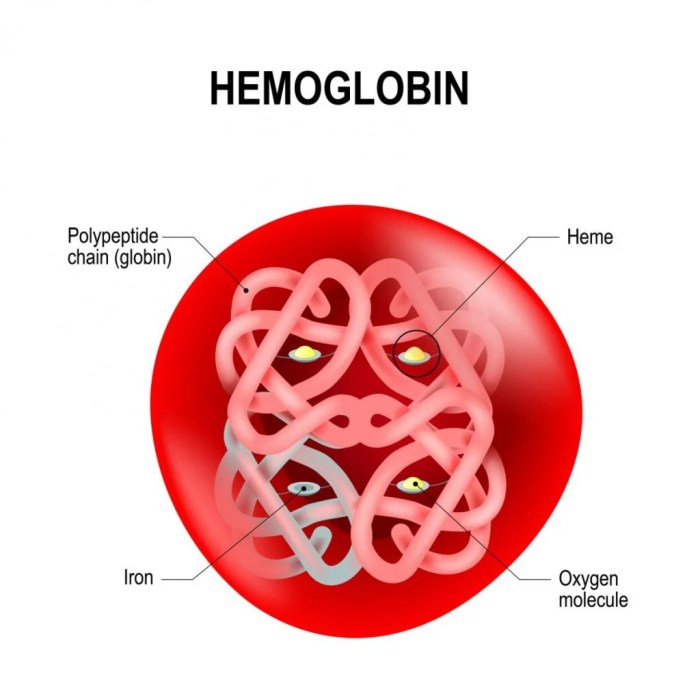
Both hemoglobin and myoglobin contain a heme group, which is an iron-porphyrin complex that binds oxygen. The heme group is located in a hydrophobic pocket in the protein, and its interaction with the surrounding amino acids affects the oxygen-binding properties of the protein.In
hemoglobin, the heme group is surrounded by a number of hydrophobic amino acids, which creates a hydrophobic environment that favors oxygen binding. In myoglobin, the heme group is surrounded by a number of hydrophilic amino acids, which creates a more hydrophilic environment that disfavors oxygen binding.
Ligand Binding, Which five statements about hemoglobin and myoglobin structure are true
Hemoglobin and myoglobin can both bind to a variety of ligands, including oxygen, carbon dioxide, and hydrogen ions. The binding of these ligands affects the oxygen-binding properties of the proteins.The binding of oxygen to hemoglobin is cooperative, meaning that the binding of one oxygen molecule increases the affinity of the protein for additional oxygen molecules.
This is due to the allosteric regulation of hemoglobin, which is mediated by the binding of ligands such as hydrogen ions and carbon dioxide.The binding of oxygen to myoglobin is not cooperative, meaning that the binding of one oxygen molecule does not affect the affinity of the protein for additional oxygen molecules.
This is due to the monomeric structure of myoglobin, which does not allow for allosteric regulation.
Evolutionary and Comparative Aspects
Hemoglobin and myoglobin are both ancient proteins that have evolved over time to optimize oxygen transport in different organisms. Hemoglobin is found in vertebrates, where it serves to transport oxygen from the lungs to the tissues. Myoglobin is found in both vertebrates and invertebrates, where it serves to store oxygen in muscle tissue.The
structures and functions of hemoglobin and myoglobin have been adapted to meet the specific needs of the organisms in which they are found. For example, the quaternary structure of hemoglobin allows for allosteric regulation, which enables it to adjust its oxygen affinity in response to changes in the environment.
This is important for vertebrates, which must be able to transport oxygen efficiently over a wide range of oxygen concentrations. The monomeric structure of myoglobin does not allow for allosteric regulation, but it does allow myoglobin to store oxygen in muscle tissue, which is important for invertebrates, which must be able to sustain activity in the absence of oxygen.
FAQ Resource: Which Five Statements About Hemoglobin And Myoglobin Structure Are True
What is the primary structural difference between hemoglobin and myoglobin?
Hemoglobin is a tetrameric protein, while myoglobin is a monomeric protein.
How does the quaternary structure of hemoglobin affect its oxygen-binding properties?
The cooperative binding of oxygen to hemoglobin’s subunits allows for efficient oxygen delivery to tissues.
What is the role of the heme group in oxygen binding?
The heme group, an iron-containing porphyrin ring, facilitates the reversible binding of oxygen molecules.
How does ligand binding affect the oxygen affinity of hemoglobin?
Ligands such as carbon dioxide and protons decrease the oxygen affinity of hemoglobin, promoting oxygen release in tissues.
What are the evolutionary adaptations that have optimized oxygen transport in different species?
Species living in low-oxygen environments often exhibit modifications in hemoglobin structure to enhance oxygen binding and transport.