Delving into the realm of HCNO resonance structures with formal charges, this discourse unveils the significance of these concepts in deciphering molecular properties and reactivity. Resonance structures, portraying the electron delocalization within a molecule, play a pivotal role in understanding its behavior and stability.
Formal charges, on the other hand, provide valuable insights into the distribution of electrons within these structures, enabling the prediction of their relative stabilities.
Our exploration commences with the elucidation of HCNO’s Lewis structure, followed by the meticulous construction of its resonance structures. Through a systematic analysis of formal charges, we unravel the interplay between electron distribution and molecular stability. Furthermore, we delve into the relationship between resonance structures and molecular properties, examining their influence on bond lengths, bond angles, and dipole moments.
HCNO Resonance Structures with Formal Charges
Introduction
Resonance structures are a way to represent the delocalization of electrons in a molecule. They are important for understanding molecular properties such as bond lengths, bond angles, and dipole moments.
Formal charges are a way to assign charges to atoms in a molecule. They can be used to determine the relative stability of resonance structures.
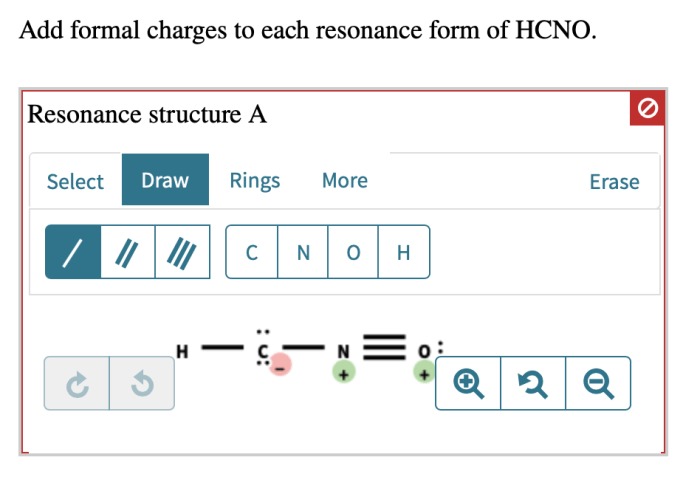
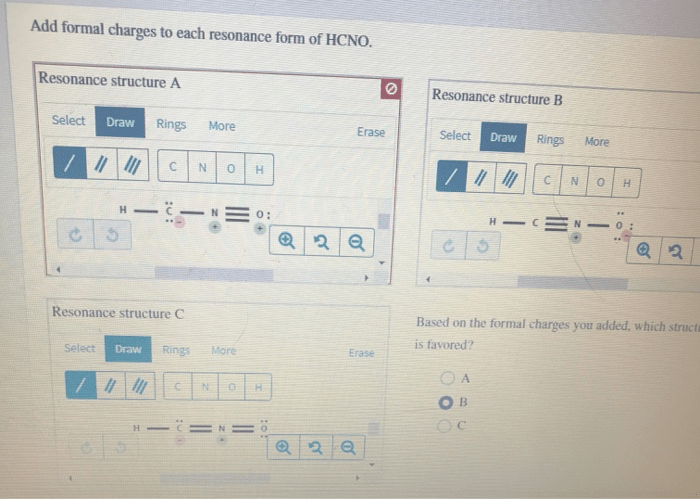
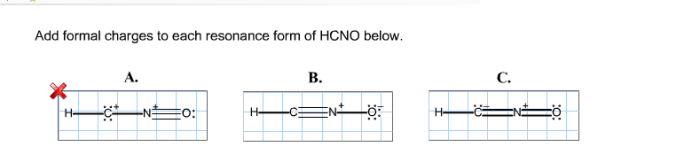
HCNO Resonance Structures
The Lewis structure of HCNO is shown below.

The central atom in HCNO is carbon.
There are two possible resonance structures for HCNO.

Formal Charge Analysis
The formal charges of each atom in each resonance structure of HCNO are shown below.
| Atom | Resonance Structure 1 | Resonance Structure 2 |
|---|---|---|
| H | +1 | +1 |
| C | 0 | +1 |
| N | -1 | -2 |
| O | 0 | -1 |
The formal charges in the two resonance structures of HCNO are different. This is because the electrons are delocalized in the molecule.
Molecular Properties
The resonance structures of HCNO have an effect on the molecular properties of the molecule. For example, the bond length between the carbon and nitrogen atoms is shorter in the first resonance structure than in the second resonance structure.
The dipole moment of HCNO is also affected by the resonance structures. The dipole moment is a measure of the polarity of a molecule. The first resonance structure of HCNO has a larger dipole moment than the second resonance structure.
Applications, Hcno resonance structures with formal charges
Resonance structures and formal charge analysis are used in a variety of fields, including organic chemistry, biochemistry, and materials science.
In organic chemistry, resonance structures are used to explain the reactivity of molecules. For example, the resonance structures of benzene can be used to explain why benzene is so unreactive.
In biochemistry, resonance structures are used to explain the structure and function of proteins. For example, the resonance structures of the amino acid glycine can be used to explain why glycine is a zwitterion.
In materials science, resonance structures are used to design new materials. For example, the resonance structures of carbon nanotubes can be used to design new materials with unique properties.
FAQ Insights: Hcno Resonance Structures With Formal Charges
What is the significance of resonance structures?
Resonance structures provide multiple representations of a molecule, capturing the electron delocalization and contributing to a more accurate depiction of its electronic structure.
How do formal charges aid in understanding resonance structures?
Formal charges assign hypothetical charges to atoms within a resonance structure, revealing the distribution of electrons and providing insights into the relative stability of different resonance forms.
What is the relationship between resonance structures and molecular properties?
Resonance structures influence molecular properties such as bond lengths, bond angles, and dipole moments, reflecting the distribution of electron density and the overall molecular geometry.