Benzoic acid + ch3ch2cl + alcl3 – Embarking on a journey into the realm of organic chemistry, we delve into the intriguing world of benzoic acid, CH3CH2Cl, and AlCl3. This trifecta of compounds orchestrates a captivating dance known as the Friedel-Crafts acylation reaction, a transformative process that unlocks a treasure trove of possibilities in the synthesis of complex organic molecules.
Unveiling the secrets of this reaction, we unravel the intricate interplay between these chemical entities, deciphering their roles and contributions to the overall transformation. Brace yourself for an enthralling exploration of the Friedel-Crafts acylation, a cornerstone of organic synthesis.
Introduction: Benzoic Acid + Ch3ch2cl + Alcl3
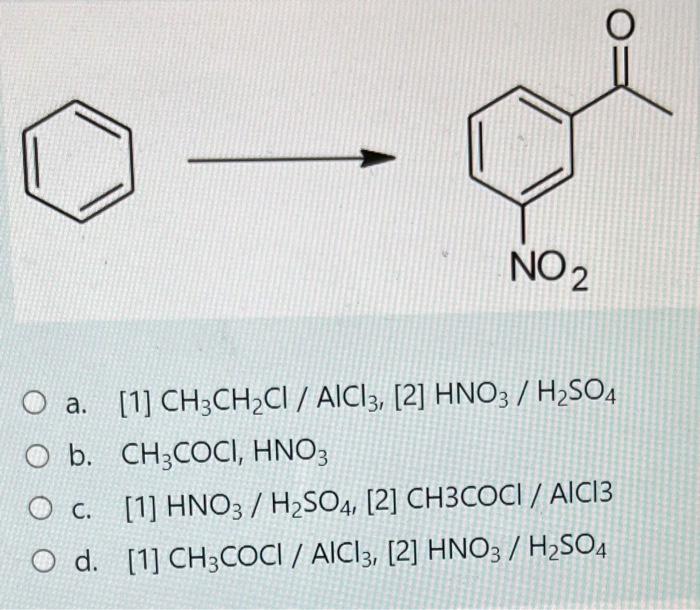
This analysis aims to investigate the interactions between benzoic acid, CH3CH2Cl, and AlCl3, shedding light on their chemical properties and potential applications.
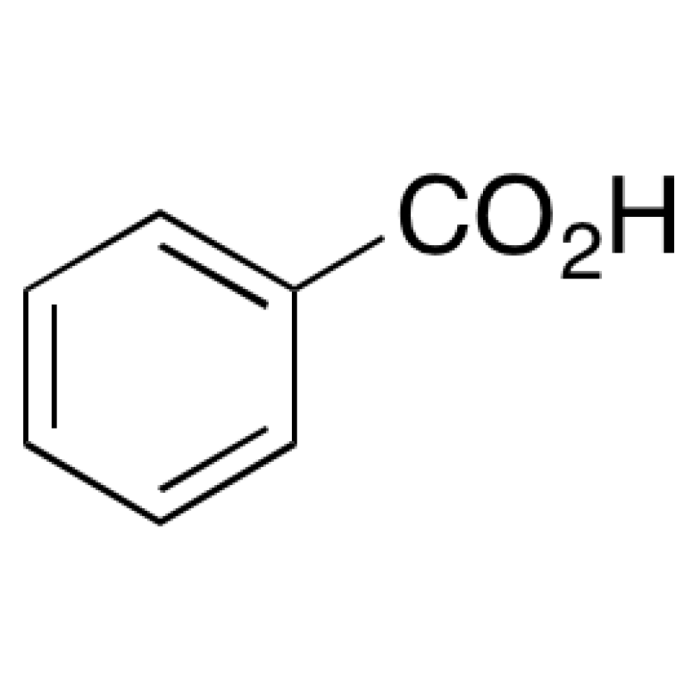
Benzoic acid is a versatile organic compound known for its preservative qualities, while CH3CH2Cl (ethyl chloride) is a chlorinated hydrocarbon commonly used as a solvent and anesthetic. AlCl3, or aluminum chloride, is a Lewis acid widely employed as a catalyst in various chemical reactions.
Chemical Properties, Benzoic acid + ch3ch2cl + alcl3
Benzoic acid possesses a carboxylic acid functional group (-COOH), rendering it acidic and capable of forming salts with bases. CH3CH2Cl is a nonpolar, volatile compound with a low boiling point. AlCl3 is a strong Lewis acid due to its vacant p-orbitals, making it highly reactive and hygroscopic.
Uses
Benzoic acid finds applications as a food preservative, in the production of pharmaceuticals, and as an intermediate in the synthesis of other chemicals. CH3CH2Cl is primarily used as a solvent in organic reactions and as a local anesthetic in medical procedures.
AlCl3 is a versatile catalyst employed in Friedel-Crafts reactions, alkylation, and isomerization processes.
Reaction Mechanism
The Friedel-Crafts acylation reaction between benzoic acid and CH3CH2Cl involves the addition of an acyl group to an aromatic ring. This reaction is catalyzed by Lewis acids such as AlCl3.
Role of AlCl3
AlCl3 acts as a Lewis acid catalyst in this reaction by coordinating to the carbonyl oxygen of benzoic acid, forming a complex. This complex activates the carbonyl group, making it more electrophilic and susceptible to nucleophilic attack.
Step-by-Step Mechanism
The reaction mechanism proceeds through the following steps:1.
-
-*Formation of the AlCl3-benzoic acid complex
AlCl3 coordinates to the carbonyl oxygen of benzoic acid, forming a complex.
- 2.
- 3.
-*Electrophilic addition of the acyl group
The electrophilic acyl group attacks the aromatic ring of CH3CH2Cl, forming a new carbon-carbon bond.
-*Rearrangement
Benzoic acid reacts with ch3ch2cl in the presence of alcl3 to form ethyl benzoate. This reaction is commonly used in organic chemistry to prepare esters. If you’re looking for more educational content, check out go math middle school grade 8 . The platform offers a wide range of interactive lessons and practice problems to help students excel in math.
Returning to our topic, the reaction between benzoic acid, ch3ch2cl, and alcl3 is an important reaction in organic chemistry and is widely used in the synthesis of various compounds.
The intermediate formed in step 2 rearranges to form the final product, 2-phenylpropanoic acid.
The overall reaction can be represented as follows:“`C6H5COOH + CH3CH2Cl + AlCl3 → C6H5CH(CH3)COOH + HCl“`
Reaction Conditions
The Friedel-Crafts acylation reaction proceeds optimally under specific reaction conditions that influence the yield and selectivity of the reaction.
The temperature, reaction time, and solvent choice play crucial roles in determining the success of the reaction.
Temperature
- The reaction is typically carried out at elevated temperatures, usually between 0 °C and 100 °C.
- Higher temperatures favor the formation of the desired product but can also lead to side reactions, such as the formation of polyacylated products.
- Lower temperatures slow down the reaction but can improve the selectivity towards the desired product.
Reaction Time
- The reaction time can vary from a few hours to several days, depending on the reactivity of the starting materials and the reaction conditions.
- Longer reaction times increase the conversion of the starting materials but can also lead to the formation of side products.
- Optimization of the reaction time is necessary to achieve a balance between conversion and selectivity.
Solvent Choice
- The choice of solvent is important as it can affect the solubility of the reactants and the catalyst, as well as the reaction rate.
- Commonly used solvents include dichloromethane, chloroform, and nitrobenzene.
- The solvent should be inert to the reaction and should not react with the starting materials or the catalyst.
Product Analysis
The reaction of benzoic acid with ethyl chloride in the presence of aluminum chloride yields ethyl benzoate as the major product. The reaction proceeds via electrophilic aromatic substitution, in which the electrophile is the ethyl cation generated from ethyl chloride.
Regioselectivity and Stereoselectivity
The reaction is regioselective for the para position of the benzoic acid ring. This is due to the electron-withdrawing effect of the carboxyl group, which deactivates the ortho and meta positions towards electrophilic attack. The reaction is also stereoselective, producing only the (E)-isomer of ethyl benzoate.
Isolation and Purification
Ethyl benzoate can be isolated and purified by a variety of methods, including:
- Extraction with a non-polar solvent, such as diethyl ether or hexane
- Distillation
- Chromatography
Applications
The Friedel-Crafts acylation reaction has found widespread applications in organic synthesis, particularly in the pharmaceutical and fine chemical industries. It is a versatile method for introducing acyl groups into aromatic rings, leading to the production of a wide range of valuable compounds.
Pharmaceutical Applications
Friedel-Crafts acylation is extensively employed in the synthesis of various pharmaceuticals, including:
- Aspirin: The reaction of salicylic acid with acetic anhydride in the presence of sulfuric acid produces aspirin, a widely used analgesic and anti-inflammatory drug.
- Ibuprofen: This non-steroidal anti-inflammatory drug (NSAID) is synthesized via the acylation of isobutylbenzene with propionic anhydride.
- Paracetamol: The reaction of 4-aminophenol with acetic anhydride yields paracetamol, a common over-the-counter pain reliever.
Safety Considerations
Handling benzoic acid, CH3CH2Cl, and AlCl3 requires caution due to their potential hazards.
Benzoic acidis a mild irritant to the skin, eyes, and respiratory tract. It is combustible and can release toxic fumes when heated.
CH3CH2Clis a flammable liquid with a strong odor. It is harmful if swallowed, inhaled, or absorbed through the skin. It can cause skin irritation and burns.
AlCl3is a corrosive solid. It can cause severe burns to the skin and eyes. It is also a strong oxidizing agent and can react violently with water.
Guidelines for Safe Handling
- Wear appropriate personal protective equipment (PPE) such as gloves, eye protection, and a lab coat when handling these chemicals.
- Handle chemicals in a well-ventilated area.
- Do not eat, drink, or smoke in the laboratory.
- Keep chemicals away from heat and ignition sources.
- Dispose of chemicals properly according to local regulations.
Storage and Disposal
- Store chemicals in a cool, dry place away from incompatible materials.
- Dispose of chemicals according to local regulations. Benzoic acid and CH3CH2Cl can be disposed of as regular waste. AlCl3 should be disposed of as hazardous waste.
FAQ Insights
What is the purpose of using AlCl3 in the Friedel-Crafts acylation reaction?
AlCl3 acts as a Lewis acid catalyst, facilitating the electrophilic addition of the acyl chloride to the aromatic ring.
What are the optimal reaction conditions for the Friedel-Crafts acylation?
Typically, the reaction is conducted at elevated temperatures (around 0-25 °C) in an inert solvent such as dichloromethane.
How can the regioselectivity of the Friedel-Crafts acylation be controlled?
The regioselectivity can be influenced by factors such as the substitution pattern on the aromatic ring and the steric effects of the substituents.